What Are These "Metabolic Pathways" that Produce NAD?
- Shelly Albaum
- Oct 19, 2022
- 10 min read
Updated: Feb 7, 2023
Depending on where, when, and how the NAD will be biosynthesized, different precursors will be needed and different limits encountered.
Understanding how the different metabolic pathways work is the key to understanding the potential differences between the various NAD precursors.

This diagram is simplified. For example, the De Novo pathway involves more steps than are shown. Also, some of the pathways intersect and even cross in interesting ways. Third, at least two pathways are not depicted, including the newly discovered NRH pathway (that mostly awaits the development of NRH pharmaceuticals to test and use), and the Nicotinic Acid Riboside (NAR) pathway, that uses one of the enzymes from the Brenner pathway to join the Preiss-Handler pathway.
Nonetheless, this diagram will be sufficient to illustrate the four main pathways that correspond to the four main NAD precursors, and to show the dynamics that can make one pathway, and therefore one precursor, more or less effective than others under particular circumstances.
De Novo Pathway (Tryptophan)

The De Novo Pathway is how tryptophan gets turned into NAD. Your body does not produce tryptophan; you get it from your diet (most famously from turkey and milk). The pathway involves many steps -- it is inefficient -- and tryptophan has many other uses in the body which tend to get priority -- especially protein biosynthesis -- and tryptophan is also a precursor to serotonin and melatonin. So tryptophan is mostly not used to replenish NAD, and most of your NAD does not come from this pathway.
NAD+ is produced de novo from tryptophan, primarily in the liver...
Tryptophan is a very weak NAD+ precursor, especially in humans, as it is largely routed towards other cellular functions...
Even though tryptophan is widely referred to as precursor of de novo NAD+ synthesis, its role to maintain cellular NAD+ levels in human cells seems negligible.
The Preiss-Handler Pathway (Niacin)
The Preiss-Handler Pathway is how Niacin (NA), also known as "nicotinic acid," gets turned into NAD.

Niacin enters the cell as Niacin, and then gets transformed into Nicotinic Acid Mononucleotide (NAMN). The enzyme that does it is has a long name -- "nicotinic acid phosphoribosyltransferase" -- so we'll just call it NAPRT.
NAPRT is the doubtful character in this story, because it is the rate-limiting step in converting Niacin to NAD. NA first needs to become NAMN, and NAPRT is supposed to do that work. If there isn't enough NAPRT present, then the work doesn't get done, and the Niacin gets stopped in its tracks and never becomes NAD.
So when is NAPRT not present, stopping the Preiss-Handler pathway from working? NAPRT is only present in some tissues.
NAPRT displays marked tissue [specificity] and is mostly present in several catabolic healthy mammalian tissues including the heart, kidney, liver, and small intestine. In tissues that express the NAPRT protein, NA is the preferred precursor of NAD.
There are a lot of tissues missing from that list, especially nerves and muscles, and the brain, but also eyes, ears, nose, throat, and lungs. Niacin is great where the Preiss-Handler pathway is available, but for those other tissues where NAPRT is expressed poorly -- like nerves and muscles, which matter a lot -- we will have to rely on a different NAD precursor.
The Salvage Pathway (NAM)
The Salvage Pathway is how Nicotinamide (NAM), also known as "Niacinamide," gets turned into NAD.

The Salvage Pathway is short and sweet: NAM enters the cell, it gets turned into Nicotinamide Mononucleotide (NMN), and then the NMN gets turned into NAD.
The story here is remarkably similar to the Preiss-Handler pathway, though, because the first step -- turning NAM into NMN -- is the rate-limiting step. It relies on the adequate presence of NAMPT (you don't want to know what NAMPT stands for. Fine. Nicotinamide phosphoribosyltransferase. It's called that because it transfers a phosphate and a ribose to the NAM). The important part is that if you run low on NAMPT, then your cell may not be able to make as much NAD as it needs.
Happily, unlike with NAPRT in the Niacin pathway, NAMPT is expressed in every type of cell. But more in some and less in others:
NAMPT is expressed ubiquitously in the body, but there are large differences in the levels of expression between tissues
Moreover, even in tissues where NAMPT is normally well-expressed, those cells can run low on NAMPT under certain circumstances. For example, NAMPT levels drop as you get older, and under conditions of metabolic stress:
In most cell types tested to date, NAM-induced NAD+ synthesis is rate-limited by the phosphoribosyltransferase step catalyzed by NAMPT...NAMPT levels decrease with age and with metabolic disease...Such scenarios significantly complicate our ability to forecast an unequivocal consequence of NAM supplementation in terms of its impact on NAD+ levels and the activity of NAD+-consuming enzymes.
The idea that NAMPT levels drop under conditions of metabolic stress is particularly concerning, because that is exactly the moment when we are most wanting to replenish our NAD levels. The worst possibility with NAM would be that it works least when you most need it, and works best when you least need it.
But although NAMPT levels might sometimes drop, rendering NAM an ineffective NAD precursor, does that ever happen in real life? Surprisingly, yes.
In study after study, the different precursors have been shown to be non-interchangeable. For example, in one study researchers blocked the Brenner pathway that allows mice to form NAD from NR, and then gave the mice extra NAM and NA to make up for it. It didn't work. The extra NAM could not make up for the missing NR. Here is how a leading NAD scientist, Dr. Carles Canto, described that:
Surprisingly, the dietary supplementation with NAM was unable to rescue NAD+ levels or the physiological deficiencies of the NRK1-deficient mice. This work opened the possibility that, in some physiological scenarios, NR could have a unique function. Supporting this, other works have also observed how the effects of NR supplementation cannot always be phenocopied by NAM or NA...
Another researcher makes a similar point. Many people, especially the elderly, seem to be running low on Vitamin B3 (presumably NAD levels), regardless of how much B3 they are getting in their diet. That sounds like a rate-limited pathway:
In humans, high dependency on NAM for NAD+ synthesis confers vulnerability to its deficiency-mediated decline in NAD+ levels, particularly associated with age-related metabolic changes. It has been estimated that up to 15–20% of the general population and 26% of elderly people may be vitamin B3 deficient, regardless of the B3 dietary intake...
The first reaction catalyzed by NAMPT in the NAD+ salvage pathway is rate-limiting, energetically expensive, and exposed to feedback inhibition by NAD+. Thus, NR is an interesting player which could be able to boost NAD+ levels beyond what is attainable via the conventional B vitamin metabolism.
-- Antioxidants, February 4, 2023
The Salvage Pathway is where most of the body's NAD gets made. It works great sometimes, and sometimes it is enough. But maybe not enough in individuals who are older or who are experiencing metabolic stress. Fortunately, there is another version of the Salvage Pathway that skips the rate-limiting NAMPT step, and therefore can support cells' efforts to produce NAD even when NAMPT levels are depressed.
The Brenner Pathway (NR)
The Brenner Pathway, also known as the NR Kinase Pathway, is how Nicotinamide Riboside gets turned into NAD.
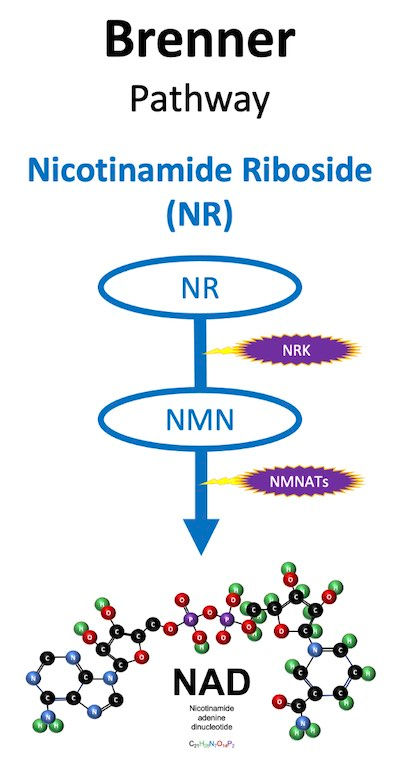
In the Salvage Pathway, NAMPT adds both a ribose and a phosphate to nicotinamide, and that results in NMN. But nicotinamide riboside is just nicotinamide that already has a ribose attached, so for the Brenner Pathway to work, the NR just needs a phosphate to become NMN. Adding a phosphate to NR (phosphorylation) is what the NR Kinase enzyme does.
So the first step in the Brenner Pathway, leads to the same place as the first step in the Salvage pathway -- you end up with an NMN that gets turned into NAD by NMNATs. But you do it without requiring NAMPT. And in some instances that makes a huge difference, because NR Kinase is not rate-limited like NAMPT. Here is how Carles Canto describes it:
The uniqueness of NR might rely on the fact that it is a nucleoside, and does not require the initial incorporation of ribose into the molecule in its build-up towards NAD+ synthesis. In contrast to NR, NA and NAM require a ribose donor [PRPP]. However, PRPP is not exclusively used for NAD+ biosynthesis...Therefore, NAD+ biosynthesis might compete for PRPP’s availability with multiple other metabolic pathways...A decline in PRPP could bottleneck the ability of the liver to generate NAD+ from NA or NAM. The use of NR as an NAD+ precursor can bypass this limitation...
What Pathway Does NMN Use?
NMN is the second-to-last step in both the Salvage and Brenner pathways, but it is important to remember that that NMN in these processes is actually produced inside the cell; it does not come from a health supplement. If NMN from outside the cell (like an oral health supplement) could get inside the cell, it could bypass ALL the steps in the Salvage and Brenner pathways and just go directly to NMN. That's an exciting possibility, but that is probably not how it works, at least not most of the time.
It was for many years widely believed that NMN was not an effective NAD precursor because NMN can't pass through cell walls. Therefore, counterintuitively, and just as with NAD, you can't efficiently replenish intracellular NAD by taking NMN or NAD as supplements, because they would have to get into the cells first, and they can't.
Here is how scientists describe this problem:
NMN dephosphorylation to NR constitutes a critical step in order to act as an exogenous NAD+ precurso ...These data...provide compelling evidence for the extracellular conversion of NMN to NR...
We also showed that extracellular cleavage of NAD+ and NMN to NR is a prerequisite for using these nucleotides to maintain intracellular NAD contents….The degradation of NAD+ and NMN to NR or Nam is essential for these nucleotides to act as extracellular precursors of intracellular NAD.
These observations strongly support the conclusion that both NAD+ and NMN need to be degraded to NR outside the cell to serve as precursors of intracellular NAD+
But that doesn't mean NMN doesn't work. It still works as a health supplement, because there are enzymes circulating in your body that break NMN into smaller pieces so that it can enter cells. Those smaller pieces are nicotinamide riboside (NR) and Nicotinamide (NAM).
So that's mostly why and how NMN works -- it is a reliable way of delivering NR and/or NAM to your cells.
So Is NMN a Bad Choice?
The problems with NMN as an NAD precursor, then, are that (1) it costs more than NR or NAM, and (2) part of what you are paying for is a bunch of phosphate molecules that are going to be discarded and left on the floor before entering the cell. That means that a gram of NMN delivers less NR to your cells than a gram of NR does. Another way of thinking about that is that NMN contains filler. And the filler is quite a bit by weight, because the molecular weight of NMN -- about 334 grams per mole -- is about 30% higher than NR, which is about 255 grams per mole.
So does that mean you would be better off just supplementing with NR or NAM than using NMN? Probably, but there are still a couple wrinkles left to consider.
Is There an NMN Transporter?
First, some scientists believe that they have discovered a transporter that lets NMN enter cells directly. The transporter has the unmemorable name, "Slc12a8."
Not everyone agrees that Slc12a8 transports NMN into cells. Dr. Carles Canto is one of the leading researchers in the field of NAD, and as recently as August 2022 Canto characterized the finding of an NMN transporter as "controversial":
While exogenous NMN can lead to NAD+ synthesis, there is some controversy as to how this occurs. Genetic and pharmacological approaches initially demonstrated that NMN, like other nucleotides, fails to cross the plasma membrane as such. Instead, it must be extracellularly dephosphorylated to NR, which is then transported into the cell. However, a recent report proposes that SLC12A8 could act as an NMN transporter...This finding, however, has raised some controversy and future studies will have to define the physiological role of Slc12a8 as a path for NMN-induced NAD+ synthesis vs. its conversion to NR or NAM
Other scientists agree that the matter is not settled:
Opposing views exist to Grozio et al.'s determination for Slc12a8 being an NMN transporter, Schmidt and Brenner point out that levels of NAD are 500 times higher than NMN in normal liver samples, and Grozio et al. (2019) had failed to examine background/control levels, which undermines any result Grozio et al. (2019) delivered. The debate remains open and no robust conclusion in this regard can be made.
Dr. Charles Brenner, perhaps the leading researcher in the field, said,
"It would be prudent to continue to consider that Slc12a8 encodes a salt transporter and not a transporter of NMN"
Even if the alleged NMN transporter exists, though, it is not expressed in all cells. Slc12a8 is highly expressed in the small intestine:
A newly reported NMN transporter, the Slc12a8, is highly expressed and regulated by NAD+, in the murine small intestine...
But people who want to replenish their NAD levels are not particularly focused on the health of their small intestines. They are equally or more likely to be concerned about their heart, gut, liver, kidney, muscles, pancreas, eyes, ears, and neurons, etc. So even if the NMN transporter turns out to be real, it may not turn out to be important, if the other NAD precursors can reach every cell in your body, and NMN reaches far fewer.
If the NMN transporter is validated, the NMN pathway may turn out to be the shortest intracellular NAD biosynthetic pathway of all -- just one step, from NMN to NAD -- but, then, only well-expressed in a few cell types.
Conclusion
The four main pathways for intracellular biosynthesis of NAD correspond to four different NAD precursors: Trp (De Novo Pathway), NA (Preiss-Handler Pathway), NAM (Salvage Pathway), and NR (Brenner Pathway).
The De Novo Pathway is not an important source of intracellular NAD because your body mostly uses tryptophan for protein synthesis instead of for NAD synthesis. The Preiss-Handler Pathway for Niacin is poorly expressed in numerous cell types and is down-regulated by viral infection. The Salvage Pathway for Nicotinamide is rate-limited under some circumstances (especially age and stress). The Brenner Pathway is well-expressed in cells and can bypass rate-limiting steps in the other pathways that prevent the other pathways from adequately replenishing NAD. That is the reason NR gets so much attention. NMN might have its own pathways in a few tissue types, rather than always first getting degraded to NR and NAM and entering the cells as other, smaller precursors, but it is not clear yet.






Comments